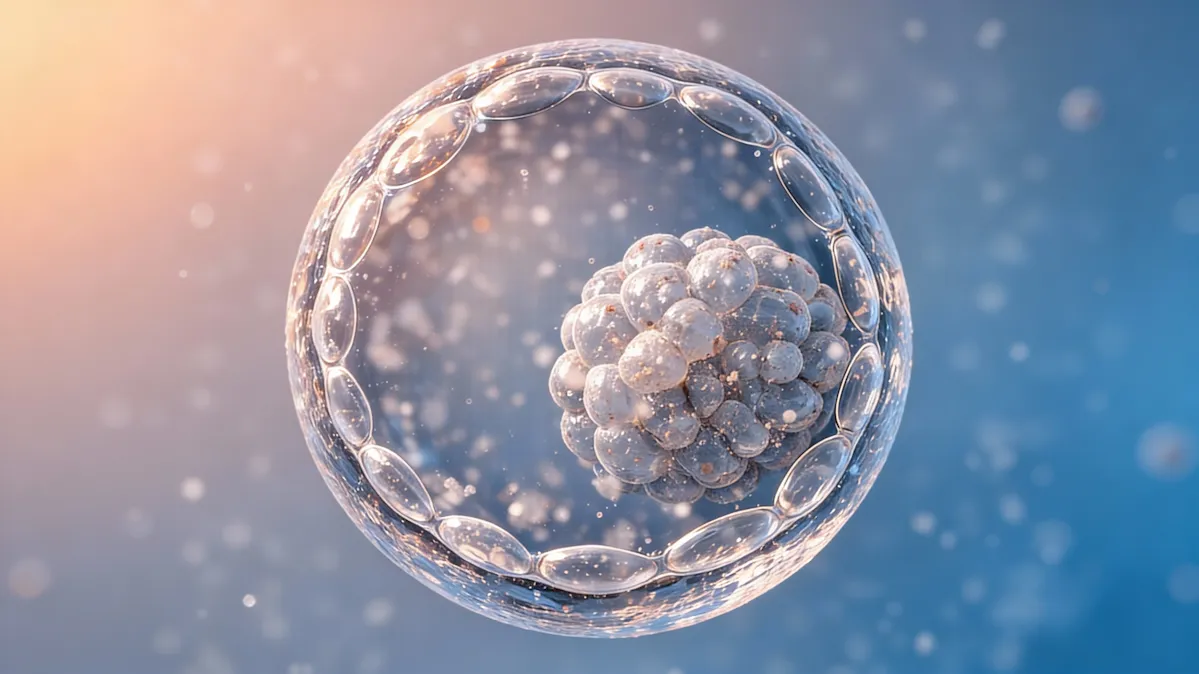
UteroPod™ is a disruptive intrauterine platform. It allows IVF-derived embryos to develop within the mother’s womb (in vivo) before proceeding to conventional selection (PGT-A) or cryopreservation.
Why the womb may enhance embryo development:
.jpeg)
.jpeg)
.jpeg)
.jpeg)
The physician collects eggs and sperm, performs ICSI (intracytoplasmic sperm injection), then carefully places the fertilized gametes inside the UteroPod — a small, porous capsule designed to allow natural uterine fluids to flow through.
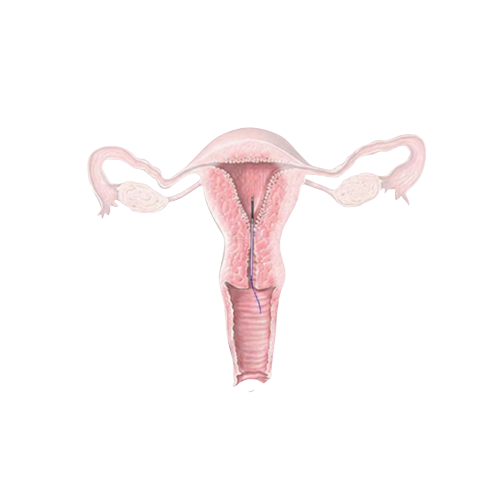
The sealed capsule is gently inserted into the uterine cavity through a simple, minimally invasive procedure — similar to an IUI or embryo transfer. From this moment forward, the embryo develops in its natural environment, the mother's uterus, rather than in a lab incubator.
Over the next few days, fertilization and early development occur naturally inside the mother's body. The embryo benefits from the uterine environment's biochemical signals and protective factors, offering natural defenses against oxidative stress during this critical phase.
After 3–5 days, the capsule is gently retrieved from the uterus. The embryos are briefly examined in the laboratory to assess their quality and stage of development. The best embryo(s) are then selected for immediate transfer back into the uterus, or they can be cryopreserved for future use.
Following transfer, the patient receives standard post-procedure care and monitoring, similar to conventional IVF. Pregnancy testing follows at the usual clinical timeline, with full medical support provided throughout the process to ensure optimal outcomes and patient well-being.
.jpeg)
The physician collects eggs and sperm, performs ICSI (intracytoplasmic sperm injection), then carefully places the fertilized gametes inside the UteroPod — a small, porous capsule designed to allow natural uterine fluids to flow through.
.jpeg)
The sealed capsule is gently inserted into the uterine cavity through a simple, minimally invasive procedure — similar to an IUI or embryo transfer. From this moment forward, the embryo develops in its natural environment, the mother's uterus, rather than in a lab incubator.
.jpeg)
Over the next few days, fertilization and early development occur naturally inside the mother's body. The embryo benefits from the uterine environment's biochemical signals and protective factors, offering natural defenses against oxidative stress during this critical phase.
After 3–5 days, the capsule is gently retrieved from the uterus. The embryos are briefly examined in the laboratory to assess their quality and stage of development. The best embryo(s) are then selected for immediate transfer back into the uterus, or they can be cryopreserved for future use.
.jpeg)
Following transfer, the patient receives standard post-procedure care and monitoring, similar to conventional IVF. Pregnancy testing follows at the usual clinical timeline, with full medical support provided throughout the process to ensure optimal outcomes and patient well-being.


Our challenge in R&D:
Our final objective With uteropods with in utero culture system is to create: A New Generation of "Hybrid Embryos" invitro/invivo As we surpass the milestone of 10 million IVF babies, we are entering the era of Enhanced Nurture.
Location & Status:
Developing at the heart of Switzerland's Health Valley.
Currently in R&D phase at Superlab Suisse, Campus Biotech (Geneva) — building preclinical data and preparing clinical validation.